Abstract
Introduction : In patients with mantle cell lymphoma (MCL), adverse events (AEs) can impair patient adherence to planned therapeutic regimens. In addition, moderate to severe AEs generally require medical attention and therefore are often associated with increased health care resource use (HRU) and costs. Real-world data on HRU and costs associated with MCL and its treatments are sparse in current literature. The goals of this study were: (1) assess HRU and direct costs of MCL; (2) examine variation in costs across treatment types; and (3) document the economic burden associated with MCL treatment-related AEs.
Methods :A retrospective cohort analysis was performed using MarketScan databases that contain employer- and health-plan-sourced administrative claims data, including information on diagnoses, procedures, and associated utilization of health care services for over 60 million privately insured individuals across the US. Cost information in the data include patient copay, coinsurance, charges, and the actual amounts paid by the payers. Patients were included if they were aged ≥18 years, had at least two medical claims on separate dates with a diagnosis code for MCL (ICD-9-CM code 200.4x or ICD-10-CM code C83.1x) during July 2010-June 2015, and were continuously enrolled in medical and drug plans for ≥12 months before the date of first MCL diagnosis (ie, study index date). Patients with evidence of MCL diagnosis or MCL treatment in the 12 months before the study index date were excluded. Mean per-patient monthly HRU and costs were assessed overall and by care setting (eg, inpatient, outpatient, pharmacy). Both all-cause and MCL-related costs were calculated; all cost data, adjusted to 2016 US dollars using the Medical Care component of the US Consumer Price Index, were assessed from the payer's perspective. The incremental HRU and costs associated with AEs were assessed by stratifying these measures according to patients' AE status (AE or no AE) and the number of AEs experienced (0, 1-2, 3-5, ≥6) during follow-up. Additionally, subgroup analyses were conducted to assess the association of AEs with mean HRU and costs among patients receiving specific regimens. All analyses were descriptive in nature.
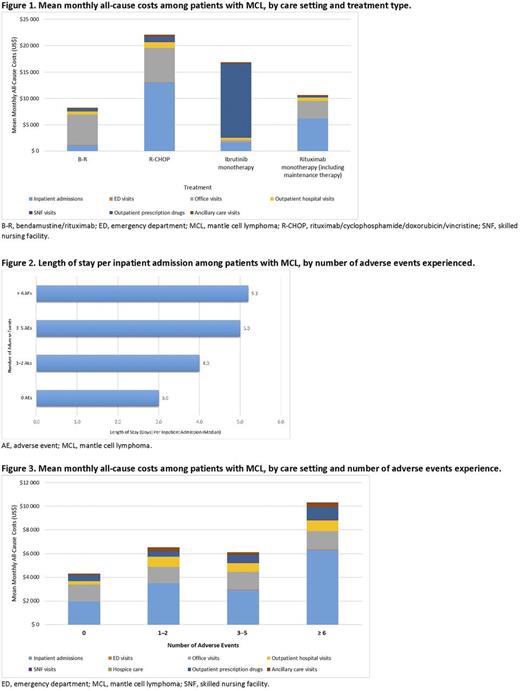
Results :In total, 783 patients with MCL (median age, 65 years [range, 19-99 years]) met the study selection criteria. Mean (standard deviation [SD]) all-cause and MCL-related monthly costs were $8393 ($16,276) and $6730 ($14,779), respectively. Inpatient admission costs ($4817 [$14,267]) and office visit costs ($1493 [$3250]) were the largest drivers of total all-cause costs. Among patients receiving systemic therapies, mean all-cause monthly costs were highest for rituximab/cyclophosphamide/doxorubicin/vincristine (R-CHOP), followed by ibrutinib monotherapy, bendamustine/rituximab (B-R), and rituximab alone (including rituximab maintenance therapy) (Figure 1); prescription drug costs were highest in patients receiving ibrutinib ($14,038 [$21,936]). The median length of stay per all-cause inpatient admission was 3.0 days (range, 2-4) among patients with no AE, which increased to 5.2 days (range, 1-50) among those with six or more AEs, as observed during all available follow-up (Figure 2). A corresponding increase in the length of stay per admission was also observed, with increasing numbers of AEs. The mean (SD) all-cause monthly costs were $4298 ($10,082) among those with no AEs and more than twofold higher ($10,335 [$16,868]) among those with at least six AEs during follow-up (Figure 3).
Conclusions :This is the largest series of patients with MCL in which real-world economic burden data have been reported. Our results demonstrate that the economic burden of MCL is substantial, with mean monthly costs varying considerably by treatment regimen and care setting. We found that inpatient admissions and office visits were the largest drivers of total costs for patients treated with R-CHOP, B-R, and rituximab, while prescription drug costs were the largest component of total costs for patients receiving ibrutinib. Patients experiencing more AEs were observed to have higher monthly costs than those experiencing few AEs.
Karve: AstraZeneca: Employment, Other: Stocks. Nagar: RTI Health Solutions: Research Funding. Goyal: RTI Health Solutions: Research Funding. Kaye: RTI Health Solutions: Research Funding. Mato: Regeneron: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Portola: Research Funding; Janssen: Consultancy; Pharmacyclics: Research Funding; DTRM: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Acerta: Research Funding; AstraZeneca: Consultancy; Kite: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal